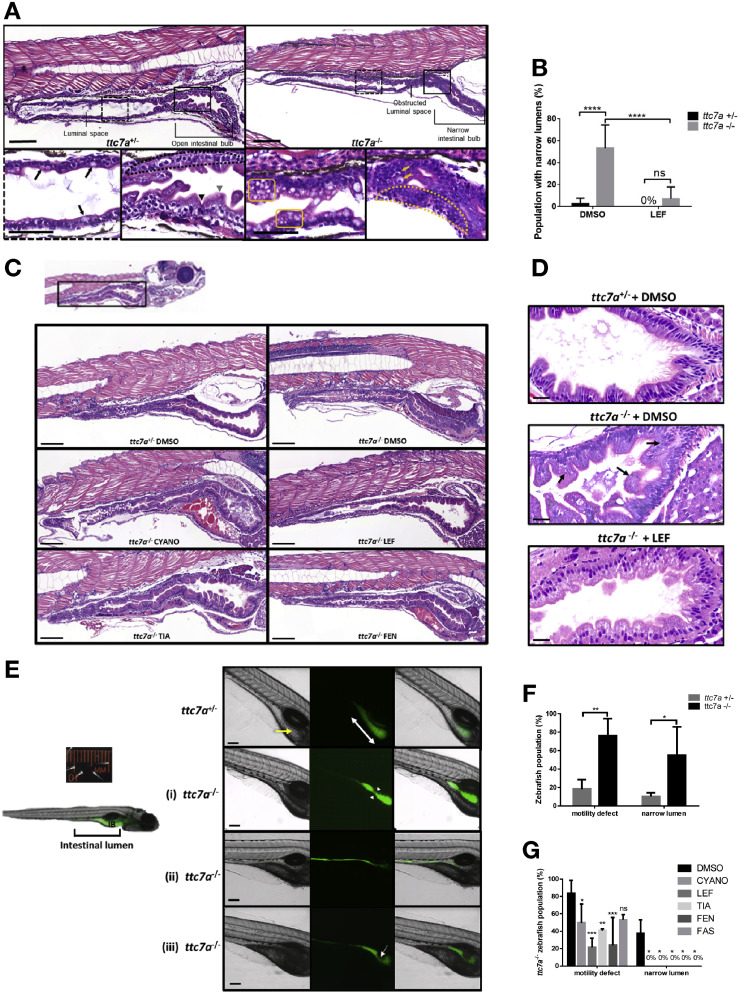
Fig. 4
Leflunomide rescues abnormal intestinal features in ttc7a–/–zebrafish. (A) Histology from ttc7a–/– zebrafish (7 dpf) shows pathologic intestinal phenotypes. Control (ttc7a+/–) zebrafish display open luminal spaces with discernible villi projections (gray arrowhead), clefts (black arrowhead), monolayer epithelium (dotted-outlined area), and mature goblet cells with large vesicles (black arrows). The ttc7a–/– zebrafish display narrowing of the intestinal bulb, stratified epithelium (yellow dotted-outlined area), signs of apoptosis (yellow arrows), and goblet cells with numerous small vesicles (yellow boxes). Original objective magnification, ×10 and ×40 for insets (scale bar, 100 μm; inset scale bar, 50 μm) (ttc7a+/–: N = 14, ttc7a–/–: N = 11). (B) Incidence of the narrow gut phenotype in DMSO- and LEF-treated fish. One-way ANOVA with post hoc test (Fisher least significant difference), ****P < .0001; (ttc7a+/–: DMSO, N = 49; LEF, N = 12; ttc7a–/–: DMSO, N = 36; LEF, N = 26). (C) Intestinal histology from treated (see Methods) ttc7a–/– zebrafish. Leflunomide, cyanocobalamin, and tiaprofenic acid suppressed the narrow-gut phenotype with minimal enterocyte crowding. Original objective magnification, ×10 (scale bar, 100 μm) (ttc7a+/–: DMSO, N = 49; ttc7a–/–: DMSO, N = 36; CYANO, N = 10; LEF, N = 26; TIA, N = 13; FEN, N = 13). (D) Assessment of apoptosis. DMSO or leflunomide treatment. ttc7a–/– zebrafish display fragmented, condensed, or engulfed nuclei in the epithelium (arrows). Leflunomide treatment resulted in diminished signs of apoptosis, reduced intestinal epithelial cell crowding, and overall improved epithelium architecture in ttc7a–/– zebrafish. Refer to Supplementary Figure 7A for the quantitation of apoptotic cells/sample. Objective magnification, ×70 (scale bar, 20 μm) (n = 6 per group, across 3 experimental clutches). (E) Staining of the intestinal lumen in control (ttc7a+/–) and ttc7a-mutant (ttc7a–/–) zebrafish. Images are from peristalsis assays (Supplementary Video 2): intestinal lumen marked by green fluorescent stain, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). The ttc7a+/– fish have discernable villi (yellow arrow) and large continuous intestinal bulbs (double-headed white arrow). Representative ttc7a–/–intestinal phenotypes (i) atresia point (white arrow heads) (ii) narrow intestinal lumen and (iii) obstruction interrupting the intestinal bulb (white arrow). Objective magnification ×4 (scale bar, 100 μm). (F) Incidence of ttc7a-mutant phenotypes. The ttc7a–/– fish have significantly larger populations with motility and narrow lumen pathologic phenotypes. Data are presented as the mean ± standard deviation; 1-way ANOVA with post hoc test (Fisher least significant difference), **P < .0054, *P < .0196 (N = 50 total for each group, across 3 experimental clutches). (G) Phenotype summary from drug-treated fish. The ttc7a–/– fish with aberrant motility and narrow lumen phenotypes were significantly reduced with cyanocobalamin, leflunomide, tiaprofenic acid, and fenbufen treatment (3–7 dpf). Data are presented as the mean ± standard deviation. Statistical significance was relative to ttc7a–/– DMSO control and determined by 2-way ANOVA with post hoc test (Dunnett) *P < .05, **P < .01, ***P < .001 (DMSO: N = 18; CYANO: N = 21; LEF: N = 21; TIA: N = 17; FEN: N = 18; FAS: N = 13 for each group across 3 experimental clutches). CYANO, cyanocobalamin; FAS, fasudil; FEN, fenbufen; LEF, leflunomide; M, mol/L; ns, not significant; RLU, relative light units; TIA, tiaprofenic acid.