Fig. 2
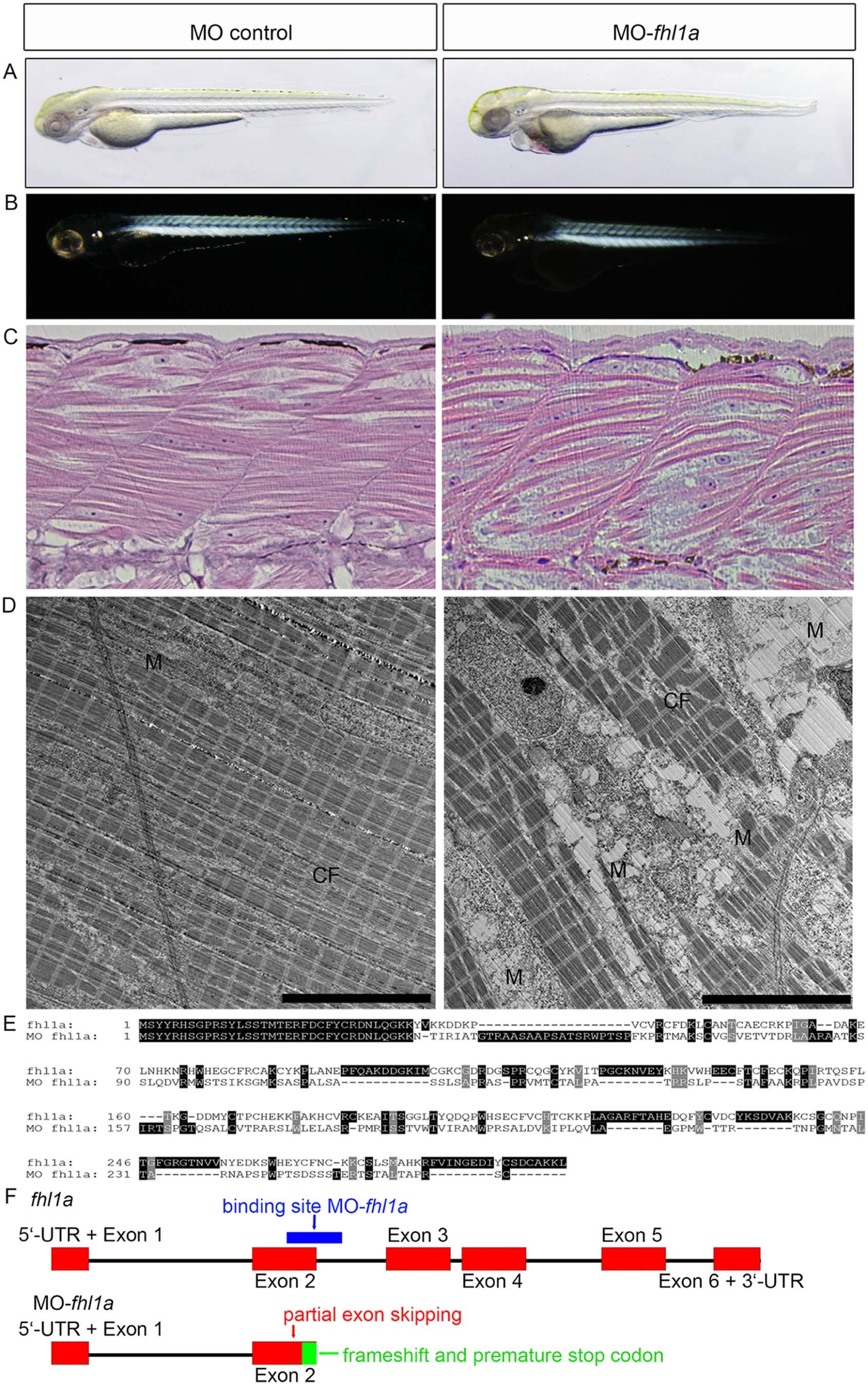
(A) Phenotype of fhl1a-morphants. fhl1a-morphants (right) display skeletal muscle myopathy and cardiomyopathy in 73% of injected zebrafish embryos (n?=?160 embryos in total, three independent experiments) at 72 hpf compared to control-injected zebrafish embryos (left). In fhl1a-morphants skeletal muscle is reduced and of irregular morphology in light microscopy. Regarding cardiomyopathy, cardiac contractility is impaired and a pericardial edema as well as blood congestion is present in fhl1a-morphants. (B) In the birefringence assay of wild-type zebrafish embryos at 72 hpf (left) the well-organized skeletal muscle appears bright. In contrast, skeletal muscle of fhl1a-morphants (right) displays a general reduction of birefringence consistent with myopathic muscle. (C) H&E staining of skeletal muscle of control-injected and fhl1a-depleted zebrafish embryos at 72 hpf. Myofibrillar disarray and a reduced content of contractile fibers in the skeletal muscle of fhl1a-morphants compared to wild-type zebrafish embryos. (D) Ultrastructural analysis by transmission electron microscopy of control-injected zebrafish and fhl1a-morphants. The content of contractile fibers (CF) is reduced in fhl1a-morphants compared to control zebrafish embryos at 72 hpf. Intermyofibrillar degeneration including enlargement of the sacroplasmic reticulum in fhl1a-morphants and subsarcolemmal and intermyofibrillar accumulation of dysmorphic mitochondria (M). (E, F) Amino acid sequence alignment and schematic illustration of Fhl1a and MO fhl1a. Morpholino-modified antisense oligonucleotide-mediated knockdown (binding site, blue) leads to a partial exon skipping of 103 nucleotides of exon 2 (red), causing a frameshift and predicted to cause an early truncation after exon 2 of Fhl1a (green region in Fig. 2F).