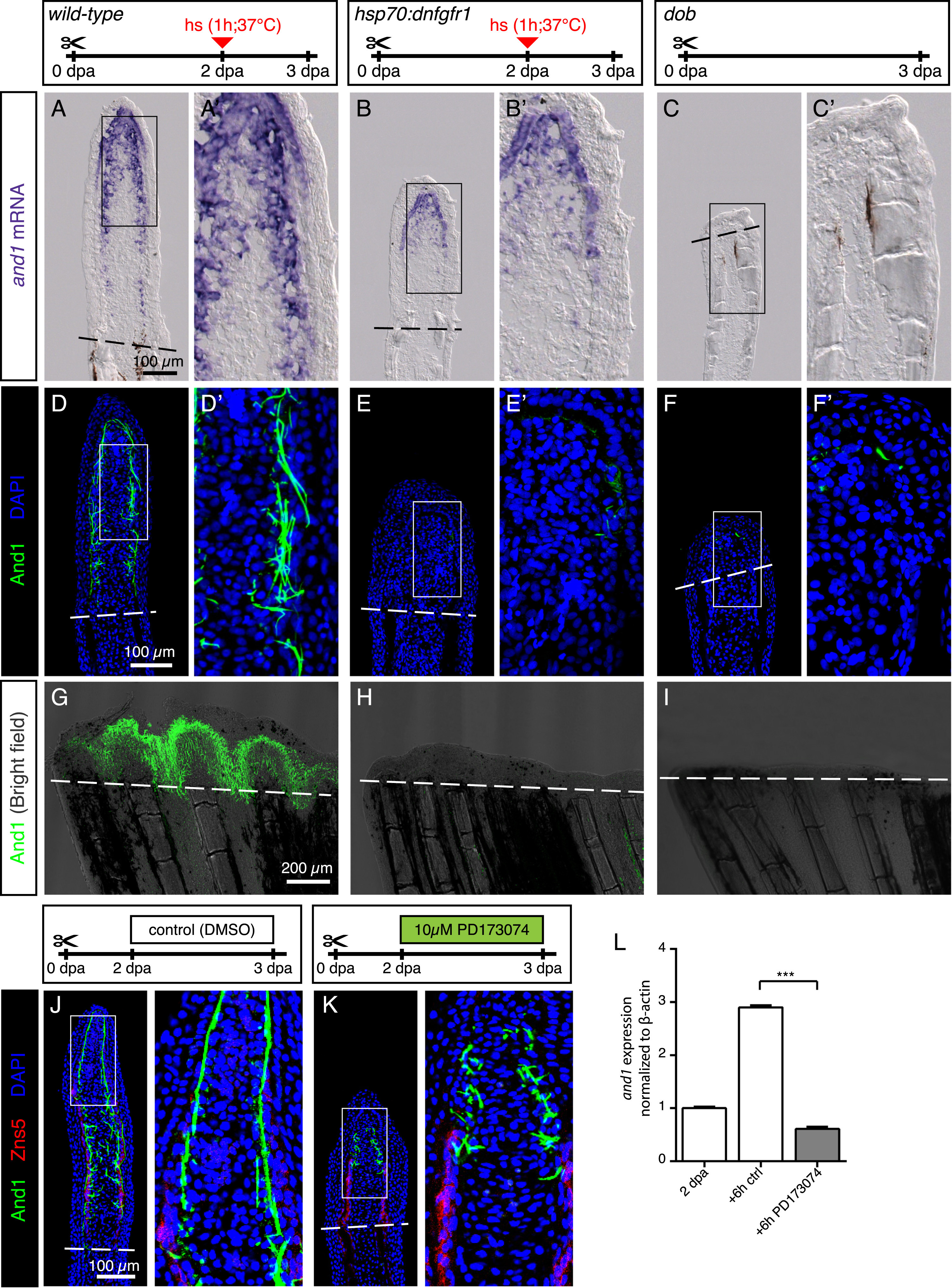
Fig. 8
FGF signaling is essential for actinotrichia formation and maintenance. (A-C) In-situ hybridization for and1 on 3 dpa sections in control, hsp70:dnfgfr1-egfp and dob fish. Control and dnfgfr1 fish underwent heat shock (hs) at 2 dpa. N = 4 fins per group. (D-F) Immunofluorescence for And1 (green) on longitudinal sections at 3 dpa in control, hsp70:dnfgfr1-egfp, and dob fish. N = 4 fins per group. (G-I) Whole-mount immunofluorescence staining for And1 (green) overlaid with bright field images for all experimental groups. N = 3 fins per group. Both, transgenic expression of dnfgfr1 and dob mutation cause a drastic decrease in and1 expression (B-C) and And1 deposition (E-F, H-I). (J, K) Immunofluorescence staining with And1 (green) and Zns5 (red) on 3 dpa longitudinal sections of fins of control fish and fish treated with PD173074 for 1 day. One day of treatment with the inhibitor of FGF signaling starting at 2 dpa is sufficient to disrupt actinotrichia in the blastema. N = 4 fins. (L) qRT-PCR analysis of and1 expression after 6 h of treatment with 10 ?M of the pharmacological inhibitor of the FGF pathway, PD173074. Treatment started at 2 dpa. The relative expression was normalized to control fins at 2 dpa. N = 3 (9 fins each). Error bars represent SEM. *** P<0.001.
Reprinted from Developmental Biology, 433(2), König, D., Page, L., Chassot, B., Ja?wi?ska, A., Dynamics of actinotrichia regeneration in the adult zebrafish fin, 416-432, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.