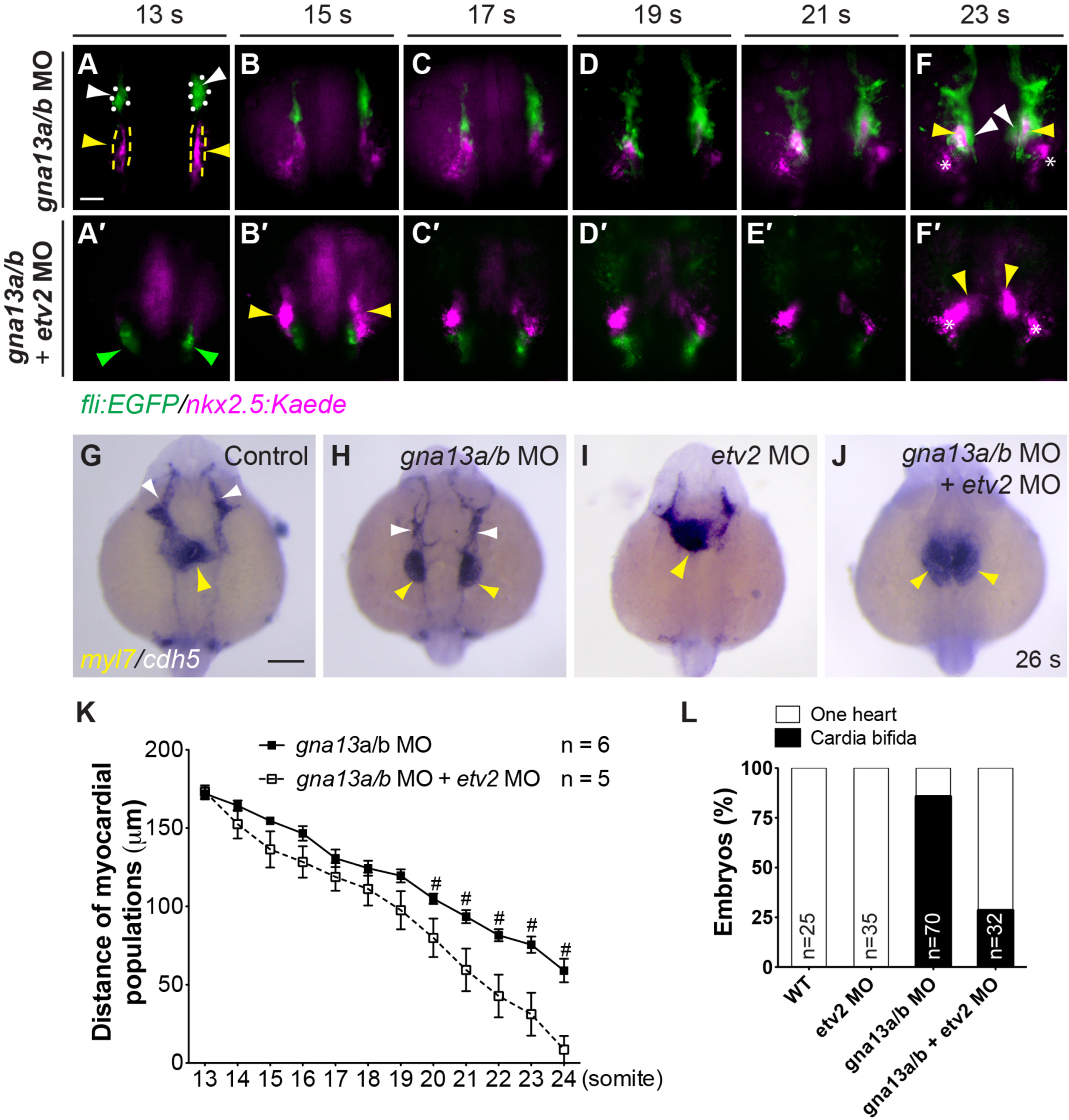
Fig. 8
Defects in myocardial migration in the context of global G?13 deficiency are partially rescued when endocardial precursors are eliminated. (A-F?) Snapshots of movies taken of Tg(fli: EGFP/nkx2.5: Kaede) embryos injected with gna13a/b MO, either alone (A-F, n=8) or with the etv2 MO (A?-F?, n=8) (Supplementary movie 5), showing the migration of endocardial (green, outlined by white dots, arrowheads) and myocardial (magenta, outlined by yellow dots and arrowheads) precursors at the stages indicated. 10 s-embryos were exposed to UV light for 1 min to convert Kaede fluorescence from green to red (presented as magenta). Asterisks: Non-myocardial cells labeled with Nkx2.5-Kaede; green arrowheads: non-endothelial cell population labeled with Fli-EGFP, which was also reported previously. Dorsoanterior views (Craig et al., 2015). (G-J) Expression of myl7 and cdh5 in 26 s embryos, as detected by ISH. Yellow arrowheads: myocardial cells; white arrowheads: endothelial cells. All images are dorsoanterior views with anterior up. (K) Distance between the two populations of endocardial cells in embryos injected with gna13a/b MO, either alone (A-F) or with the etv2 MO (A?-F?), at indicated stages. Data are meanħs.e.m. #: P<0.05 between the two experimental groups. (L) Frequencies of heart defects (two separated and fused hearts) in the indicated embryos in G-J. The number of embryos in each sample is shown. Scale bars: 100 µm.
Reprinted from Developmental Biology, 414, Xie, H., Ye, D., Sepich, D., Lin, F., S1pr2/G?13 signaling regulates the migration of endocardial precursors by controlling endoderm convergence, 228-43, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.