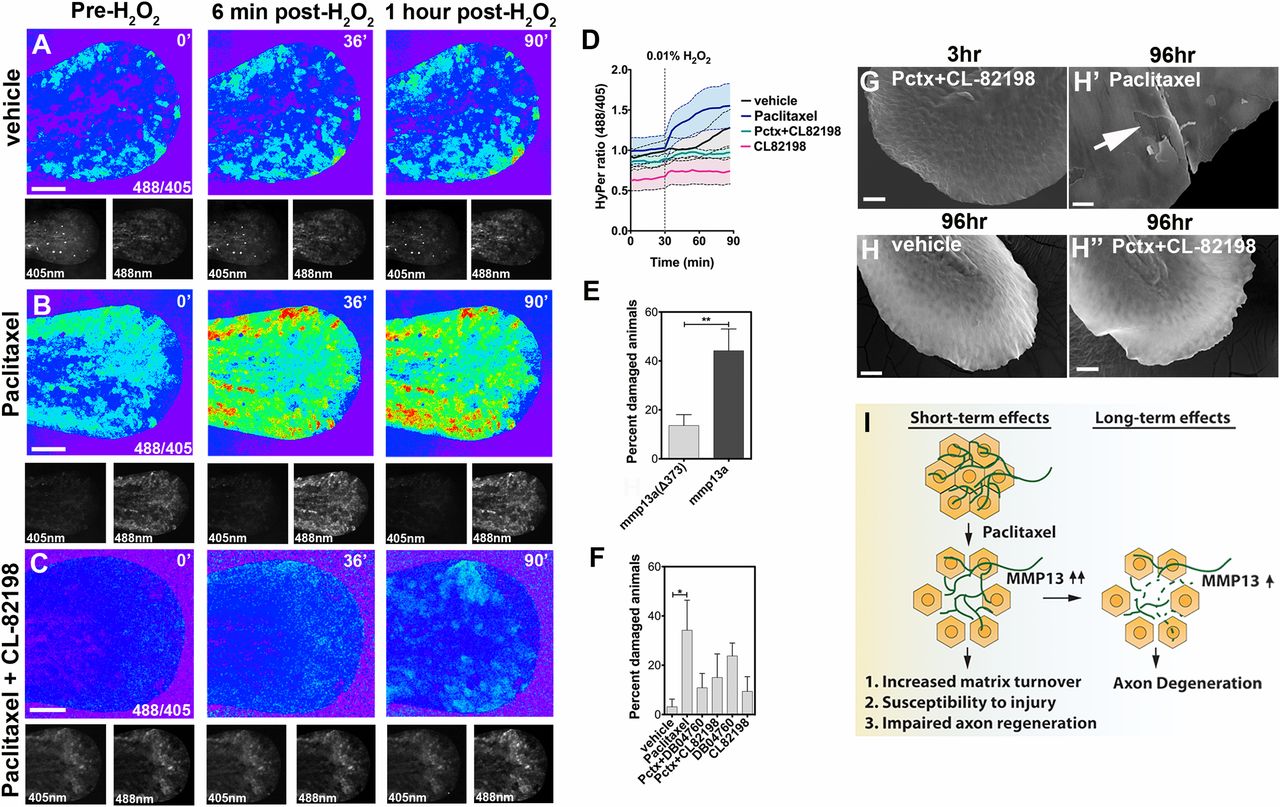
Fig. 7
Epithelial defects induced by paclitaxel are rescued upon MMP-13 inhibition. (A-C) Temporal sequence of HyPer oxidation in Tg(krt4:Gal4_tdTomato_5xUAS_HyPer) larva before and after addition of 0.01% exogenous H2O2 at 30 min, visualized as 488/405 nm emission ratio. Vehicle (0.5% DMSO) controls show some oxidation following H2O2 addition (A), which is increased after 3 h of paclitaxel incubation (B) and rescued when CL-82198 is coadministered (C). (Scale bar, 100 µm.) (D) Quantification of HyPer oxidation (n = 2, 4-5 fish per group; paclitaxel vs. paclitaxel + CL-82198; *P = 0.03). (E) Percentage of larvae with skin damage following injection of either wild-type mmp13a or mmp13aΔ373 mRNA into one-cell stage embryos and mechanical stress at 2 dpf (n = 3 biological replicates, 15 larvae per group). (F) Rescue of skin damage following pharmacological inhibition of MMP-13 and mechanical stress at 2 dpf (n = 3, 9-10 larvae per group). (G-H′′) SEM of larvae incubated for 3 h in paclitaxel + CL-82198 (G) and 96 h in vehicle (H), paclitaxel (H′), or paclitaxel + CL-82198 (H′′) shows improved skin morphology with CL-82198. [Scale bar, 25µm (G, H, and H′′) and 5 µm (H′).] (I) Model of paclitaxel-induced peripheral neuropathy. Paclitaxel damages epithelial keratinocytes by up-regulating MMP-13, leading to skin damage due to increased matrix turnover and neurotoxicity. *P < 0.05, **P < 0.01. Pctx, paclitaxel.