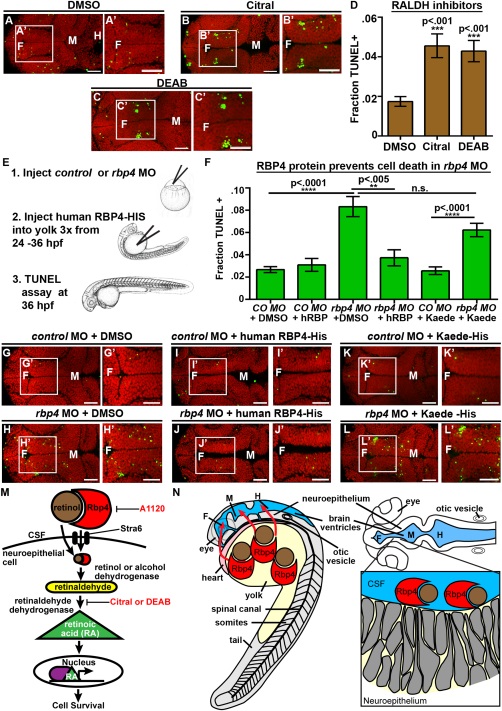
Fig. 7
RA synthesis is required for cell survival and Rbp4 can be transported from the yolk to the CSF. (A–C) Dorsal view of TUNEL (green) and propidium iodide (red) in embryos injected with DMSO (A), Citral (B) or DEAB (C). (D) Quantification of TUNEL after injection of inhibitors into wild-type embryos every two hours from 22 to 36 hpf. (E) Experimental design for injection of human RBP4 protein into yolk of rbp4 morphants. (F) Quantification of TUNEL positive cells in rbp4 morphants after injection of RBP4 or Kaede protein. (G–L) Dorsal view of TUNEL (green) and propidium iodide (red) in control MO + p53 MO (G,I,K) or rbp4 MO+ p53 MO (H,J,L) embryos injected with DMSO (G,H), human RBP4-His protein (I,J) or Kaede-His protein (K,L). (M) Model of retinoid signaling pathway in the embryonic brain. Inhibitors used in red. (N) Model of Rbp4 + RA precursor transport. Rbp4 bound to retinol/RA precursors can be transported from the yolk to CSF (left, red arrows) where it is taken up from the CSF by a RA metabolizing cell, oxidized into RA and promotes cell survival (right). Data represented as mean ± SEM. F = forebrain, M = midbrain, UP = unpunctured, P = punctured, D = drained. Scale bars = 50 µm.