Fig. 3
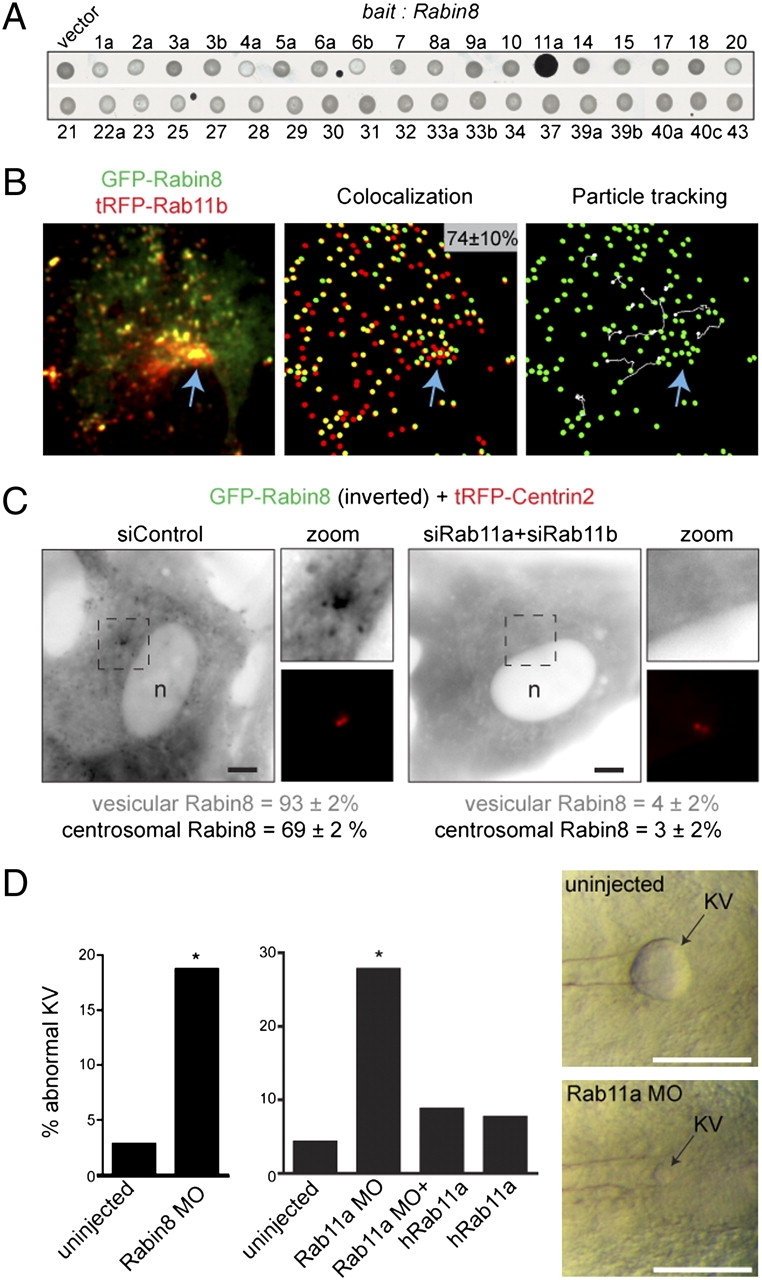
Rab11 is required for Rabin8 vesicle recruitment and centrosomal trafficking and is important for ciliated KV formation in zebrafish. (A) Rabin8 specifically interacts with the GTP-locked form of Rab11a (Q70L) by yeast two-hybrid analysis. Rabin8 was screened against a library of 37 constitutively active (?GTP-locked?) Rabs. One isoform for most of the 60 known Rabs was included (exceptions were Rab24, -26, -35, -36, and -38). (B) GFP-Rabin8 and tRFP-Rab11b vesicles colocalize and are transported to the centrosome. (Left) RPE GFP-Rabin8 cells transiently expressing tRFP-Rab11b (48 h) were serum starved for 1 h and imaged by live dual-view SDC microscopy. (Center) In five cells analyzed, 74 ± 10% of GFP-Rabin8 vesicles (green spheres) colocalized with tRFP-Rab11b structures (red spheres). (Right) Particle-tracking software was used to visualize the trajectory (shown in white) of Rab11b-Rabin8 double-positive vesicles. Blue arrows mark the pericentriolar region. (C) Rab11 is required for Rabin8 trafficking. Micrographs show representative siRNA-treated RPE GFP-Rabin8 cells expressing tRFP-Centrin2 that were serum-starved for 1 h. GFP-Rabin8 vesicle recruitment and centrosomal accumulation was scored in live cells (n = 50). Representative results from three independent experiments are shown. (Scale bar: 5 μm.) (D) Rabin8 and Rab11a knockdown caused defects in KV formation in zebrafish. Embryos were untreated or injected with 5 ng Rabin8 MO and 2.5 ng Rab11a MO as previously described (4). (Right) Micrographs show KVs from 8?10 somite embryos. (Left) Rabin8 MO caused KV abnormalities in 19% of embryos (29/155; P < 0.0001), compared with >4% of control embryos (3/108). (Center) Rab11 MO caused KV abnormalities in 27% (38/137; P < 0.0001) of embryos, compared with >4% of control embryos (3/71). Coinjection of hRab11a mRNA rescued the phenotype (9%, 11/126) in Rab11a MO-treated embryos (Fig. S3 J and K).