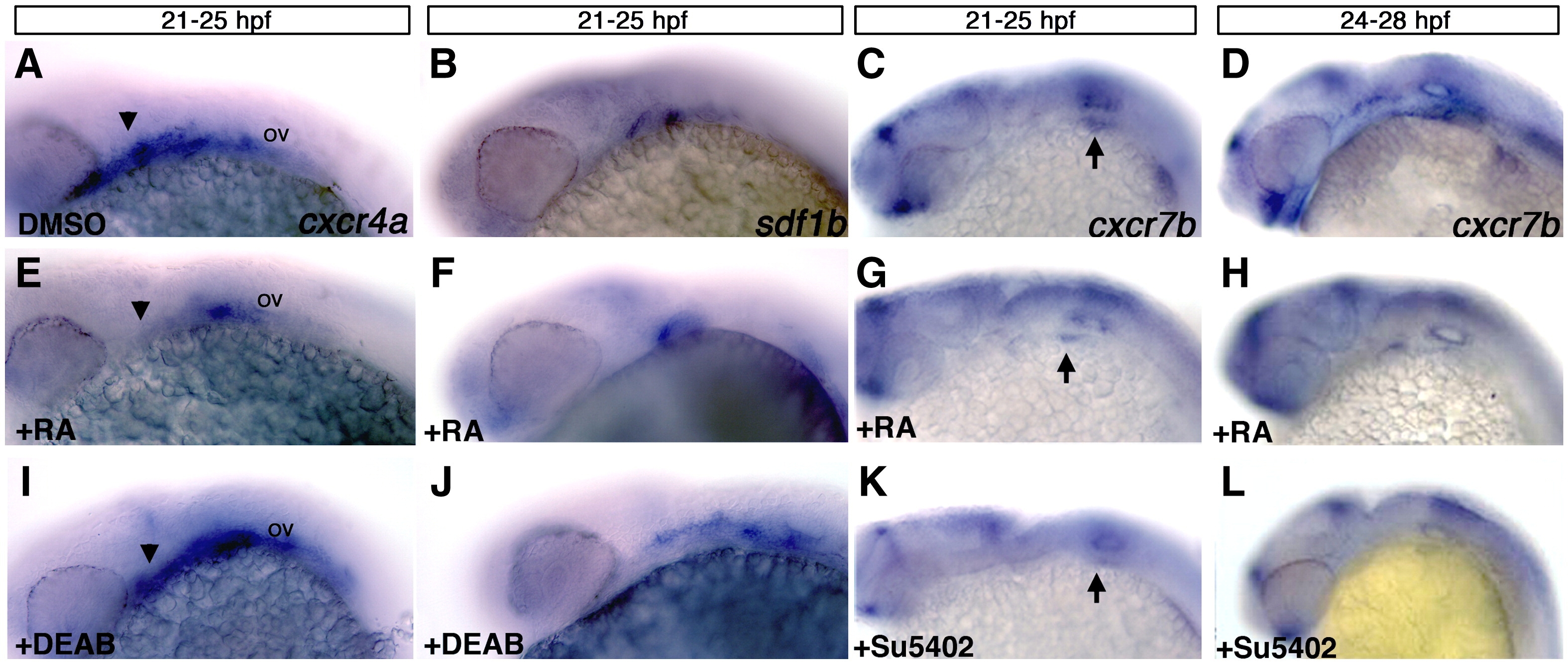
Fig. 6 Retinoic acid signaling is upstream of cxcr4a and sdf1b. Lateral views, anterior to the left of 25 hpf (A?C, E?G, I?K) and 28 hpf embryos (D, H, L). (A?D) Control embryos treated with DMSO between 21 and 25 hpf show normal expression of cxcr4a (A), sdf1b (B), and from 21?25 hpf and 24?28 hpf showing normal cxcr7b expression (C, D respectively). Embryos treated with 100 μM RA from 21?25 hpf show reduced expression of cxcr4a (E as compared to A; arrowheads), a mild reduction in pouch 2 of sdf1b (F) and mild reduction of cxcr7b (G) below the otic vesicle (arrows point to otic vesicle region in C, G, K). Treatment from 24?28 hpf (H) causes a reduction of cxcr7b expression in the endoderm but not the forebrain as compared to wildtype embryos. Embryos treated with the 50 μM RA synthesis inhibitor DEAB show an expansion of cxcr4a (I) and sdf1b (J) expression posterior to the otic vesicle (ov) as compared to DMSO treated embryos in (A, B). Embryos treated with the Fgf inhibitor Su5402 from 21?25 hpf (K) and 24?28 hpf (L) have reduced expression of cxcr7b in the otic region (K, L) and within the pharyngeal endoderm (L) as compared to DMSO treated controls (C, D).
Reprinted from Developmental Biology, 333(1), Olesnicky Killian, E.C., Birkholz, D.A., and Artinger, K.B., A role for chemokine signaling in neural crest cell migration and craniofacial development, 161-172, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.