- Title
-
Modeling Sarcoglycanopathy in Danio rerio
- Authors
- Dalla Barba, F., Soardi, M., Mouhib, L., Risato, G., Akyürek, E.E., Lucon-Xiccato, T., Scano, M., Benetollo, A., Sacchetto, R., Richard, I., Argenton, F., Bertolucci, C., Carotti, M., Sandonà, D.
- Source
- Full text @ Int. J. Mol. Sci.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
EXPRESSION / LABELING:
PHENOTYPE:
|
|
Swimming ability of the |
|
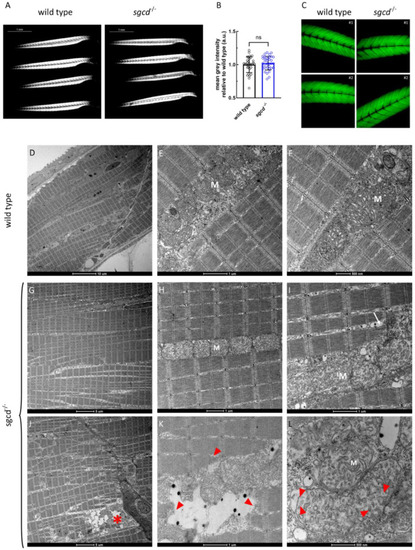
Muscle fiber integrity at the macroscopic and ultrastructural level in the |
|
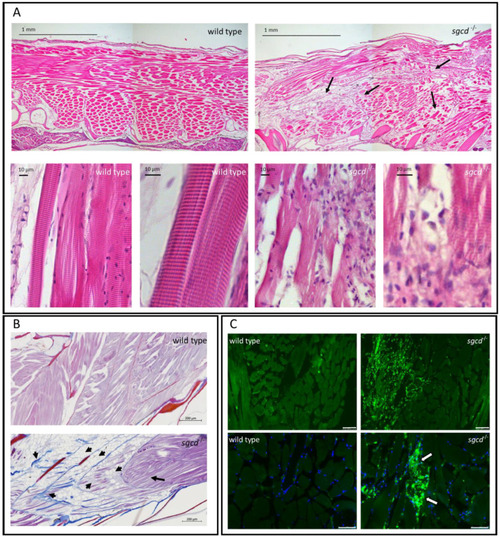
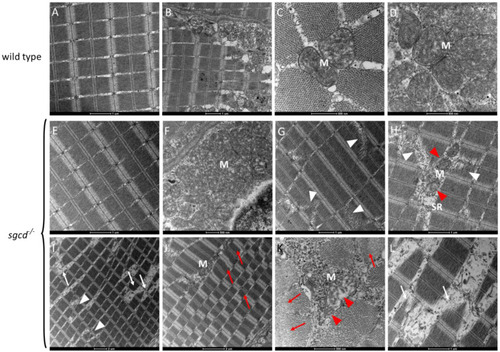
δ-SG deficiency causes defects in the skeletal muscle of adult zebrafish. ( |
|
Transmission electron microscopy analysis of the skeletal muscle of the WT and PHENOTYPE:
|
|
Swimming performance of the adult wild type and PHENOTYPE:
|
|
Muscle fiber integrity in the representative wild type and PHENOTYPE:
|
|
The E262K mutant of δ-SG is the substrate of the ERAD in zebrafish. ( EXPRESSION / LABELING:
|